Application - Anti-cancer antibody repertoires and checkpoint inhibition
In recent years, the study of tumor-infiltrating lymphocytes (TILs) has uncovered many new functionalities and ambivalent roles of these cells in the tumor. TILs not only contain a potential to kill cancer cells but also to shape the tumor microenvironment together with cancer cells, which in turn influences exerted immune functions and the tumor itself. While past studies have mainly focused on T cells, recent findings suggest a more prominent role for tumor-associated antigen (TAA) specific B cells and their multifunctionality in tumor infiltrates and in tumors exhibiting tertiary lymphoid structures. Indeed, antigen presentation can lead to antibody-secreting cells (ASCs) producing TAA-specific antibodies capable of directly targeting tumors. Nonetheless, there is also evidence for autoreactive antibodies in patient sera, suggesting the existence of a cross-reactive antibody repertoire. This requires the host to carefully balance antibody specificity to allow for tumor binding without emerging autoimmune side-effects.
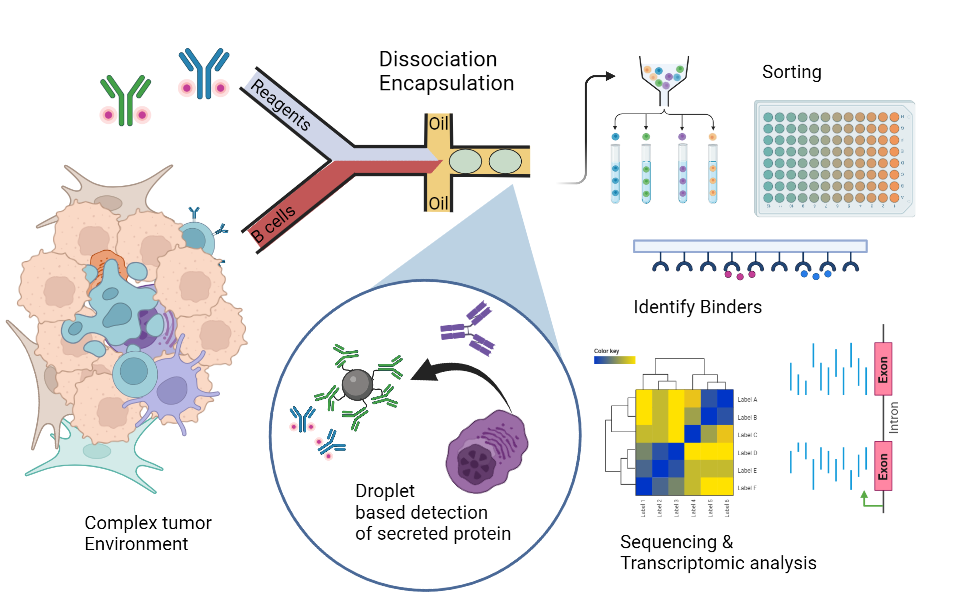
In this project, we aim to investigate the complex relationship between antibody secretion of TAA and self-specific B cells. Using single-cell antibody secretion assays and tumor-antigen cDNA expression libraries, we will characterize clonality and cellular functionality of ASCs from different compartments of a murine pancreatic tumor model. Ultimately, we aim to link how memory and plasma cell differentiation towards a tumor-associated phenotype affects the antibody repertoire. Applying this knowledge to settings of cancer immunotherapy may further explain the role and molecular profile of B cells in cancer as well as elucidate the mechanisms behind their ambivalence in functionality
Several student projects and internship opportunities are available. If you are interested, please contact with a short statement of motivation and CV.
References:
1. Eyer, K., Doineau, R.C.L., Castrillon, C.E., Briseño-Roa, L., Menrath, V., Mottet, G., England, P., Godina, A., Brient-Litzler, E., Nizak, C., Jensen, A., Griffiths, A.D., Bibette, J., Bruhns, P., Baudry, J., 2017. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat. Biotechnol.
2. Mazor, R. D. et al. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell 185, 1208-1222.e21 (2022).
Mirlekar, B., Wang, Y., Li, S., Zhou, M., Entwistle, S., De Buysscher, T., Morrison, A., Herrera, G., Harris, C., Vincent, B.G., Ting, J.P.-Y., Rashid, N., Kim, W.Y., Yeh, J.J., Pylayeva-Gupta, Y., 2022. Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity. Cell Rep. Med.
3. Sharonov, G.V., Serebrovskaya, E.O., Yuzhakova, D.V., Britanova, O.V., Chudakov, D.M., 2020. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol.
4. Schumacher, T.N., Thommen, D.S., 2022. Tertiary lymphoid structures in cancer. Science
Contact
Funktion. Immunrepertoireanalyse
Vladimir-Prelog-Weg 1-5/10
8093
Zürich
Switzerland