Technology - Bioassay development and the science of measuring
Interested in measuring a certain functionality on the single-cell level? We have established around 50 different assays on the single-cell level, so chances are good that we might measure your analyte of interest. Please contact us.
Measuring functionality on the single-cell level is a challenge due to the minute amount of proteins, metabolites and other molecules one cell produces. Furthermore, large heterogeneity can be present at the single-cell level. In the example of antibody secretion, a small subset of cells is able to produce thousands of antibodies per second, whereas others do not or only secrete tens of antibodies/s.
To be able to quantify functionality, an integral part of our work is to develop assays that enable us to quantify functionality in a manner that is compatible with high-throughput screenings and consequently allow the characterization of the heterogeneity of immune cells. Development is followed by thorough characterization and validation of these novel bioassays that are often leaning towards simplified version of physiologically relevant processes. The goal of this assay development is to remove complexity without sacrificing relevance and information.
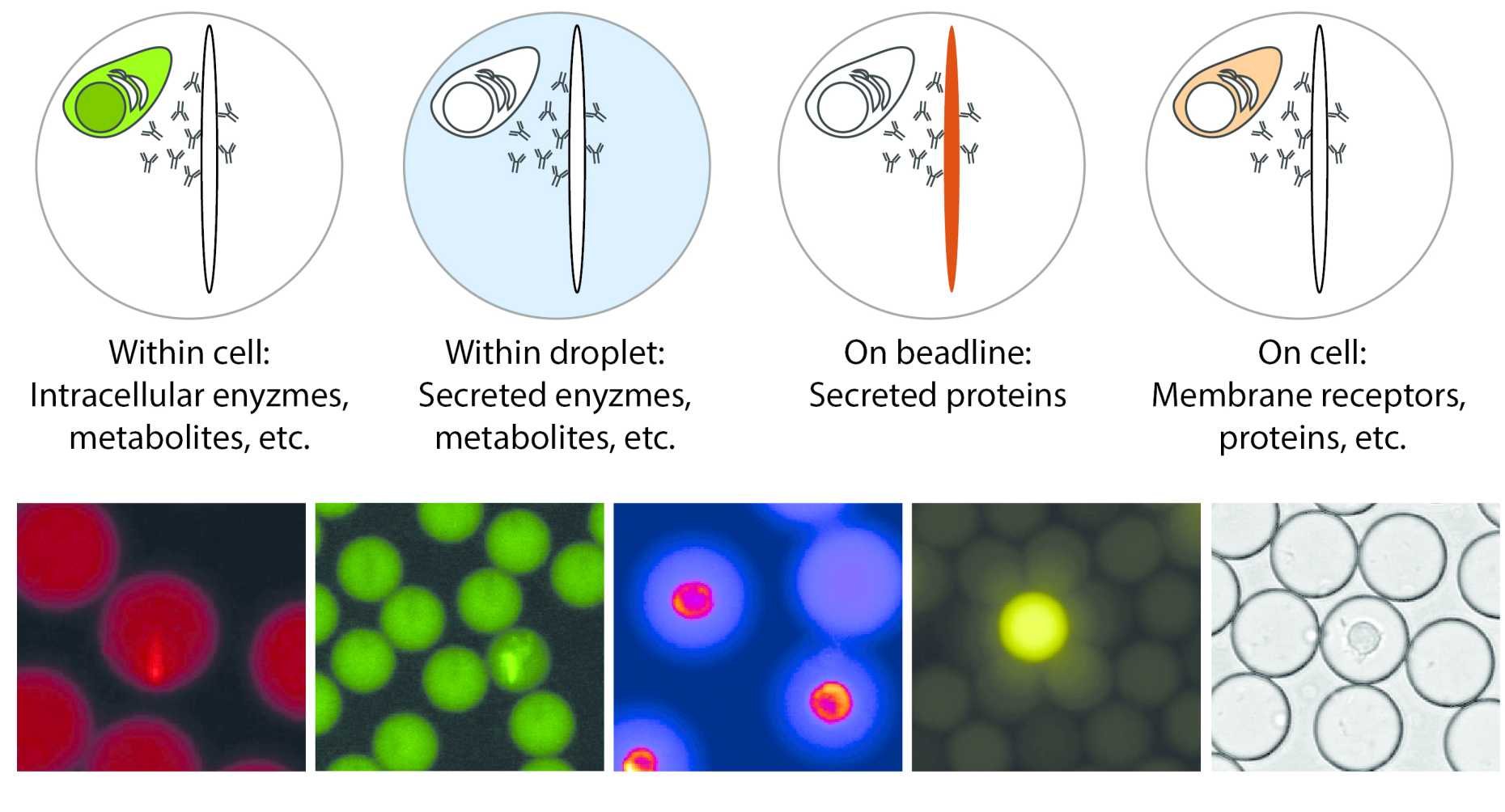
Since many functionalities in the immune system are controlled by secreted and soluble factors, such as cytokines, chemokines, cytotoxic enzymes or antibodies, we focused first on the development of methods to quantify these secreted factors. To quantify secreted proteins, we developed an in-droplet sandwich immunoassay based on paramagnetic nanoparticles (see also list of publications). To perform these assays, the paramagnetic nanoparticles are pre-coated with a capture reagent against the target of interest – usally an antibody that binds either a specific cytokine, chemokine or other antibodies.
How does it work: Within each droplet, a few hundred to thousands of these nanoparticles are encapsulated (depending on the expected secretion rates), and by applying a magnetic field the particles are induced to form an elongated, observable aggregate, we termed beadline. The use of nanoparticles increases the capacity of the employed assays and adds additional flexibility to determine the measeurement range. If the cell now produces the analyte of interest, the secreted protein will bind to the beadline, and relocate a fluorescently labelled detection antibody that is also present in the droplet. Therefore, the resulting fluorescence relocation can be read-out as an increase in fluorescence on the beadline, normalized by the background fluorescence in the droplet, and can be calibrated using known protein standards to relate the measured signal to concentration of the analyte. Since we can follow the increase in concentration of the analyte in each droplet over time, the secretion rate becomes accessible for each cell indivdiually. In addition, the frequency of secreting cells can be extracted by automatic counting of droplets with relocation above a certain threshold, and are measured as a fraction of all present cells.
This assay has been used to quantify the secretion or shedding of up to 20-40 different markers, ranging from different antibody isotypes, and a variety of murine and human cytokines (including TNF-α, IFN-γ; IL-2 and IL-6), to shedded and cleaved membrane proteins. All of these secreted molecules are quantified using a fluorescence relocation-based, sandwich immunoassay as described above. To identify cells and characterize membrane bound effector molecules, a similar relocation assay can be designed, measuring relocation (and therefore protein concentration) on the cell membrane. Alternatively, the droplet volume can also be used for biochemical reactions, such as for the measurement of secreted, small metabolites where no antibody pairs can be found – such as lactate, ATP or other. Several fluorescence channels can be used to measure different analytes, and up to 5-6 different functionalities can therefore be measured in parallel, allowing to correlate various functionalities to the individual cell. We are constantly designing new assays to study novel functionalities, re-develop and adapt current assays and are also working on strategies to increase this number to enable even more complex correlative studies of functionality.